ETRI Increases Energy Density by 20% with Novel-Concept Rechargeable Battery Design
- Novel-concept cell design without a current collector, lowering battery weight and increasing energy density
- Design applicability strengthened through eco-friendly and low-cost water-based process application
A group of South Korean researchers has developed a cell design technology to improve the energy density of rechargeable batteries by approximately 20%. This technology is expected to contribute significantly to implementing rechargeable batteries with high energy density through battery weight reduction.
Electronics and Telecommunications Research Institute (ETRI) has presented a novel rechargeable battery design with the current collector1) removed. The removal of the current collector reduces the rechargeable battery weight. As a result, energy density, which is a performance based on weight, is stably improved.
To validate its excellence, this technology was published online in Advanced Energy Materials2), a top-tier international journal in the field of energy materials, on October 22.
Used in our daily lives and across industries, such as for smartphones, electric vehicles and unmanned aerial vehicles, rechargeable batteries are considered one of the 12 national strategic technologies. In particular, energy density of a rechargeable battery is a key indicator for evaluating the battery performance in terms of its significant impact on durations of use, installation areas, etc. of devices equipped with the battery.
Accordingly, studies for materials are being actively conducted in order to improve the energy density of rechargeable batteries. However, factors such as the non-uniformity of mineral deposits, the scarcity of certain elements and price fluctuations based on the supply and demand of resources pose major obstacles. This has caused difficulty in stably bringing rechargeable batteries with improved energy density to the market.
1) Current collector: A thin film inside a rechargeable battery measuring 10 to 20 micrometers (ten-thousandths of a centimeter) in thickness that has low electrical resistance and is configured to allow the movement of electrons during the rechargeable battery charge or discharge
2) Advanced Energy Materials: IF (24.4), JCR by sector (top 2.65%), https://onlinelibrary.wiley.com/doi/full/10.1002/aenm.202403655
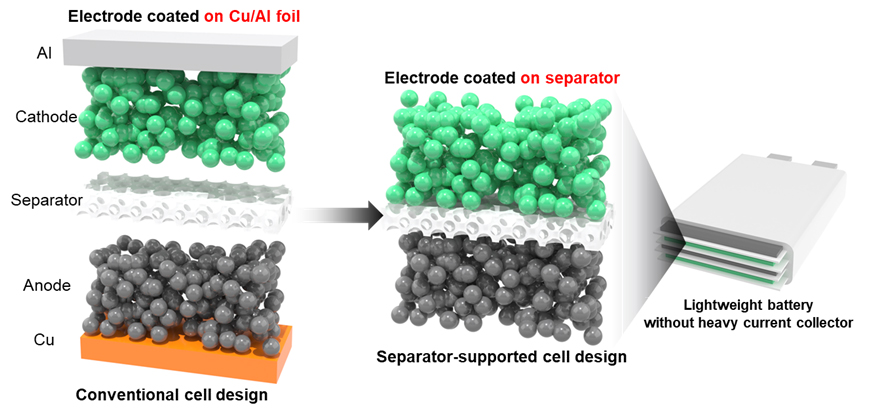
Conventional Battery Structure (left), Newly Developed Battery Design without a Current Collector (right)
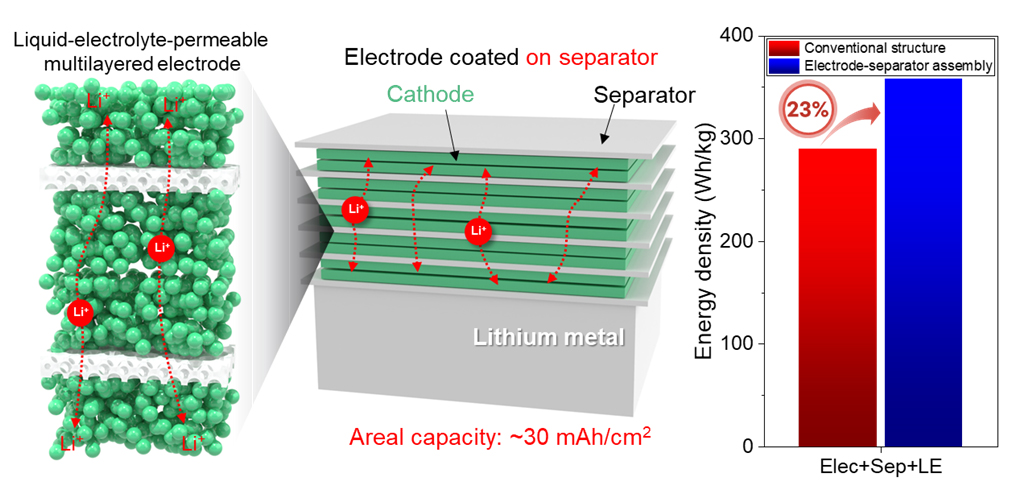
Implementation of Battery with High Energy Density through Multilayering of the Newly Developed Electrode
To address these challenges, ETRI researchers have proposed a way to improve energy density while reducing material dependence. They devised and validated a new design approach that completely eliminates a current collector from the rechargeable battery.
The current collector plays the role of providing abundant electrons for electrochemical reactions at the cathode and anode during the rechargeable battery charge or discharge. However, the high density of a current collector has caused an increase in the battery weight. As a result, industry-related researches have focused on minimizing the thickness of current collectors.
ETRI researchers have devised an innovative electrode design, where the electrodes are directly coated onto the separator without a current collector. The design has been established as an environment-friendly and cost-competitive water-based process1), further enhancing its applicability.
1) Water-based process: This refers to a manufacturing process using water. Organic solvents are commonly used in electrode fabrication processes. However, due to high process costs, environmental concerns, and worker health and safety issues, water-based binders and eco-friendly solvents, or dry fabrication processes are being actively studied.
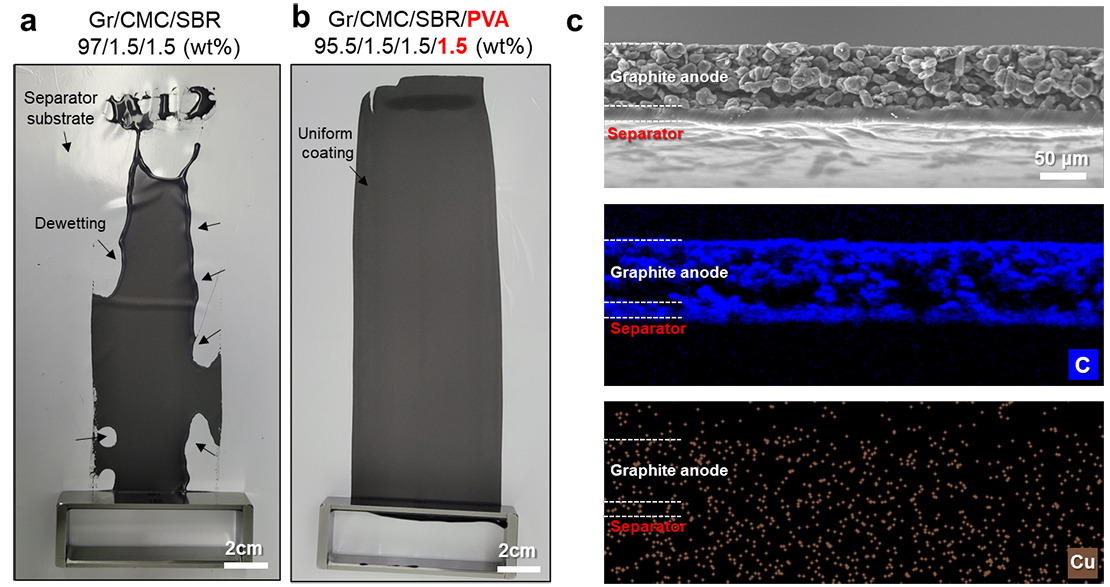
Evaluation of Coatability on Separator According to Polyvinyl Alcohol Content in Electrode Slurry (left) & Result of Electron Microscopy Analysis (right)
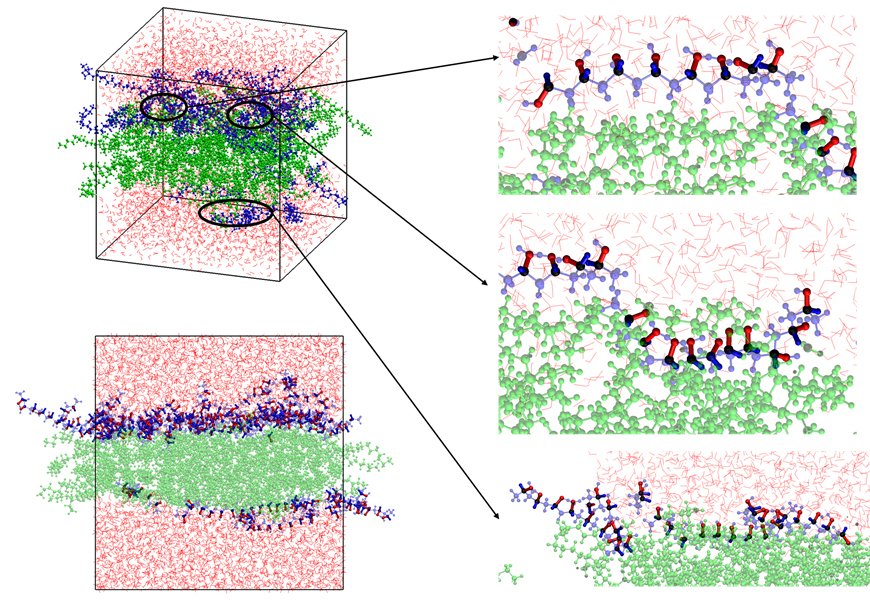
Result of Molecular Simulation for Interaction between Polyvinyl Alcohol & Separator
Moreover, a polyvinyl alcohol polymer binder1) is used in the water-based process to uniformly apply electrodes to the separator with a low wettability to water. Through joint research with Korea University, the researchers theoretically verified the improvement of interfacial stability2) using the polymer.
In addition, unlike conventional structures, the proposed electrode structure allows electrolytes to pass through smoothly, which enables a new type of battery design with multilayered electrodes. The researchers have confirmed that the energy density of the rechargeable batteries can be improved by nearly 20% through this design.
The new electrode design was also found to improve the safety of the separator, increase recyclability of the electrodes, and facilitate the analysis of electrochemical reactions within the electrode.
1) Polyvinyl alcohol polymer binder: A polymer with the structure of [CH2CH(OH)] repeating, highly soluble in water
2) Interfacial stability: Stabilizing the interfacial properties of a hydrophobic separator surface by making it hydrophilic to allow the smooth application of aqueous slurries

ETRI Senior Researcher Ju Young Kim who led the research explained, “This is the culmination of our efforts to develop a type of battery platform that can be utilized in improving the energy density of rechargeable batteries. This research is particularly meaningful for taking place through the Next-Generation Research Program to discover and support upcoming and promising researchers at ETRI.”
The Next-Generation Research Program is independently operated by ETRI in order to discover and foster innovative ideas of young and creative researchers. In this program, which was launched last year, six and seven tasks were selected and supported in 2023 and 2024, respectively.
Going forward, the researchers plan to expand on their findings to introduce a rechargeable battery with further improved energy density. They will also continue conducting research on the electrode designs to concurrently produce high energy density and power output.
Based on the ETRI Next-Generation Research Program, this research was undertaken with support from the Global TOP Strategy Research Group and Phase-Advancing Phase Leap Type Carbon-Neutral Technology Development Project led by the Ministry of Science and ICT.
Led by ETRI, the research was jointly undertaken with the research teams of Prof. Yong Ju Kim at Korea University and Prof. Yong Min Lee at Yonsei University. The first authors of the research paper are Senior Researcher Ju Young Kim of ETRI and Researcher Min Young Seo at Korea University.
Ju Young Kim, Senior Researcher
Smart Materials Research Section
(Tel. 82-42-860-6423, juyoung@etri.re.kr)
 Previous
Previous